$$ D E T O N A T I O N $$
|
--button_text--
interessant
|
|
witzig
|
|
gut analysiert
|
|
informativ
|
0
Dear Shareholders:
We are very pleased to have successfully completed the merger of Laiyang Jiangbo Pharmaceuticals Co., Ltd. ("Laiyang Jiangbo") with Genesis Technology Group, Ltd. -- resulting in a new public company called Genesis Pharmaceuticals Enterprises, Inc. I want to take a moment to reach out to all of our shareholders in order to review where we are, how we got here and what shareholders may expect in the future.
I am pleased to report that the final terms of the merger were completed on October 1, 2007. As a result of the merger, Genesis Pharmaceuticals Enterprises, Inc. is listed on the OTC Bulletin Board and trades under the symbol "GTEC."
The new company is a pharmaceutical company whose primary operations are those of our operating subsidiary Laiyang Jiangbo. Founded in 2003, Laiyang Jiangbo focuses on the research, development, production, marketing and sales of new and sophisticated pharmaceutical products in the People's Republic of China. We have rapidly established ourselves as a leader in drug development and modern marketing in China, and our lead drugs enjoy significant market share in their categories.
Our company has a very strong financial profile
For its fiscal year ended June 30, 2007, Laiyang Jiangbo reported revenue of US$76.2 million, up by 55% over the prior year. Gross profit increased by 64% to US$55 million, and our operating income increased by 53% to US$18.3 million, over the same period. For fiscal 2007, we reported audited net income of US$22.1 million, inclusive of a one-time US$9.9 million credit for a corporate and VAT tax exemption we received from the Chinese government. Our fiscal condition is also very solid, with total assets of US$55.4 million, shareholders' equity of US$27.3 million, and cash on hand of US$17.7 million, as of June 30, 2007.
Our company is well positioned in the very attractive China pharmaceutical market
Pharmaceutical demand in China is forecast to expand by more than 13% annually to reach about US$51 billion by 2010. Strong economic growth, changing demographic patterns, and the reform and expansion of health care systems are expected to drive greater demand for drugs. Genesis Pharmaceuticals is well positioned to meet that increased demand.
Our current products are approved by the Chinese State Food and Drug Administration and include Clarithromycin sustained-release tablets, Itopride Hydrochloride granules, Ciprofloxacin Hydrochloride tablets and Paracetamol tablets. We started sales of Baobaole chewable tablets this month, in November.
We have a strong and growing pipeline of Class 1 drugs
A research and marketing driven organization, our company is driven to bring new drugs to market that can significantly improve clinical outcomes for patients in major disease categories. Since 2005, our three major drugs (Ciprofloxacin Hydrochloride, Paracetamol and Clarithromycin) have been on the approved list for reimbursement by the national healthcare Insurance Catalogue. A fourth drug (Itopride Hydochloride) is expected to get provincial government approval soon, which will make it available to over 166 million potential users. We aim to have all of our new drugs under development be on government approval lists. This is very important to maximize the availability of drugs to patients and revenue generating potential of our products.
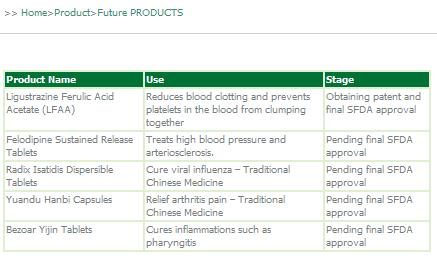
Genesis recently announced a US$5 million private placement financing for the acquisition of a new Chinese Class 1 drug Ligustrazine Ferulic Acid Acetate ("LFAA"), a cardiac cerebral vascular drug that is designed to help reduce blood clotting and prevent platelets in the blood from clumping together. Assuming that LFAA receives all of the necessary Chinese regulatory approvals, the Company plans to put the drug into trial production in fiscal year 2008, and expects fiscal year 2009 sales of US$9.9 million and fiscal year 2010 sales of US$26.3 million.
We are adding new drugs to our product line to increase overall growth for the company. We are currently seeking government approvals to produce Felodipine Sustained Release Tablets, Radix Isatidis Dispersible Tablets, Yuandu Hanbi Capsulesa and Bezoar Yijin Tablets. Drug sales have a lifecycle during which competition often leads to decreasing profit margins over time. Our focus going forward is on Chinese Class 1 drugs that often have high profit margins. Manufacturing these sophisticated new drugs requires advanced technology, which means competitors face high barriers to entry. We are committed to having and using the latest technology to manufacture new sophisticated drugs with high profit margins.
Genesis Pharmaceuticals Enterprises, Inc. will continue to grow by expanding its distribution network of 440 full time and 620 part time representatives throughout China. Sales to end users such as doctors and hospitals, and not just distributors, help build brand recognition and customer loyalty. Sales representatives will continue to sell existing and new products to existing and new customers.
Our team is committed to operate at world class standards.
We have a strong management team in place, which has recently been enhanced by the addition of a qualified Chief Financial Officer with U.S. GAAP experience, Elsa Sung. Most of Genesis Pharmaceuticals' senior management has extensive industry experience and has been in place since the founding of Laiyang Jiangbo in 2003. They will ensure that Genesis Pharmaceuticals executes its growth strategy.
Now, after completing our going public transaction, we are committed to developing the human resources and systems required to meet our responsibilities as a U.S. public company. We are committed to high quality disclosure and shareholders will be able to observe management activities and obtain accurate information about the Company through the company's website (http://www.genesis-china.net/), which will soon be relaunched in both English and Chinese.
We are committed to the highest level of corporate governance. Our new Board of Directors consists of high level managers and independent Directors:
Cao Wubo Chief Executive Officer and Chairman of the Board
Xu Haibo Vice President, Chief Operating Officer and Director
Feng Xiaowei Outside Director
Huang Lei Outside Director
Ge Jian Outside Director
Zhang Yihua Outside Director
Rodrigo Arboleda Outside Director (From Genesis Technology Group)
Robert Cain Outside Director (From Genesis Technology Group)
Over the next several quarters we plan to take the steps required to move Genesis Pharmaceuticals to a major stock market in the U.S. so as to attain greater visibility with investors and the media and enhance the marketability of our stock.
Genesis Pharmaceuticals' senior management plans to take a trip to the U.S. in December 2007. This trip will give U.S. shareholders a chance to meet with management and ask management questions about Genesis Pharmaceuticals.
I hope that this letter has given our new shareholders some sense of the development of our company and our strategic direction. Our team looks forward to updating you on our progress through regular conference calls and visits to meet with our investors. In closing, I would like to thank our customers, shareholders, directors and employees for the Company's present and future success. Thanks to all of you for your continuing interest in Genesis Pharmaceuticals.
Cao Wubo, Chief Executive Officer and Chairman of the Board
Genesis Pharmaceuticals Enterprises, Inc.
CONTACT: Genesis Pharmaceuticals Enterprises, Inc.
Ms. Elsa Sung CFO
+1-877-895-3650, Ext. 701
info@Genesis-China.net
CCG Elite Investor Relations, Inc.
Mr. Crocker Coulson, President
+1-646-213-1915 (New York)
crocker.coulson@ccgir.com
Source: PrimeNewswire (November 20, 2007 - 12:50 PM EST)
News by QuoteMedia
www.quotemedia.com
Optionen
| Antwort einfügen |
| Boardmail an "_bbb_" |
|
Wertpapier:
EUTEX EUROPEAN TELCO
|
0
Wednesday November 21, 8:30 am ET
LAIYANG, China, Nov. 21, 2007 (PRIME NEWSWIRE) -- Genesis Pharmaceuticals Enterprises, Inc. (OTC BB:GTEC.OB - News) (``Genesis'' or the ``Company''), a U.S. pharmaceutical company with its principal operations in the People's Republic of China, today announced that it signed a cooperation agreement with The Institute of Microbiology, Chinese Academy of Sciences (IMCAS), a research institution in China that performs a wide spectrum of basic and applied research in the field of microbiology.
ADVERTISEMENT
According to the cooperation agreement, Genesis and IMCAS will build a ``Genesis Pharmaceuticals Industrialization Model'' to do joint research with the goal of commercializing pharmaceutical discoveries. This Industrialization Model is IMCAS' first cooperative arrangement with a pharmaceuticals manufacturing company.
Genesis will fund the Industrialization Model's daily operations and research and development activities. Once new drugs have completed a first stage of experiments in the Industrialization Model, they will be delivered to Genesis's factory for further testing to determine the viability of commercial wide-scale production. Genesis' research and development staff will work with researchers from IMCAS to resolve issues that arise during the process of designing the manufacturing process for new drugs. Genesis will have the first right to purchase patents for any products developed by the Industrialization Model and IMCAS.
``We are very pleased that IMCAS chose Genesis to be its partner in the commercialization of new drug discoveries,'' said Mr. Cao Wubo, Chairman and CEO of Genesis Pharmaceuticals Enterprises, Inc. ``Because IMCAS is the national leading research institution in the field of microbiology, we believe collaboration will bring valuable research and develop expertise to Genesis and strengthen our own R&D force. This will extend our company's use of cutting edge technologies as well as solidify our leading position in the Chinese pharmaceutical market.''
About IMCAS
The Institute of Microbiology, Chinese Academy of Sciences (IMCAS) was founded on December 3, 1958. It is a national comprehensive research institution that performs a wide spectrum of basic and applied research in the field of microbiology. IMCAS hosts 300 faculty and staff, including 5 CAS academicians. Currently, it has three key laboratories: State Key Laboratory of Microbial Resources, State Key Laboratory of Plant Genomics, and CAS Key Laboratory of Systematic Mycology and Lichenology as well as nine research centers which carry out studies on microbial resources, microbial genomics, agricultural biotechnology, bio-energy and industrial biotechnology, environmental biotechnology, extremophiles, microbial metabolic engineering, molecular virology, and molecular immunology, As the national leading research and development organization in China, IMCAS successfully developed SARS vaccine.
As the national leading research institution in the field of microbiology, the IMCAS is committed to the advancement of science and technology. During the last 40 years, it has attained a number of developmental achievements in molecular evolution, pathogenicity, and cross-species propagation, as well as antiviral fields which can be the initial force for industrialization process.
Optionen
0
Genesis Pharmaceuticals Launched Sales of Baobaole Chewable Tablets
LAIYANG, China, Dec. 21, 2007 (PRIME NEWSWIRE) -- Genesis Pharmaceuticals Enterprises, Inc. (OTCBB:GTEC) ("Genesis" or the "Company"), a leading pharmaceutical company in the People's Republic of China, today announced that it had launched its newest product Baobaole chewable tablets for sale throughout China.
Baobaole chewable tablets are a traditional Chinese medicine used to treat gastric and general abdomen discomfort. This drug stimulates appetites and promotes digestion with mild and lasting effects. It is a new, non-prescription over-the-counter (OTC) drug. A nationwide marketing campaign for Baobaole chewable tablets was started in August 2007 that included regularly shown television advertisements on the CCTV Economic Channel and Movie Channel. The Company's sales force will distribute the drug.
"We are optimistic about the launch of this product into the rapidly growing Chinese OTC market. This is our first large scale operation in the over-the-counter drug market as well as the non-prescription medicine market. We hope that this product introduction will strengthen Genesis' overall market presence and increase our market share of domestic drug sales," said Mr. Cao Wubo, Chairman and CEO of Genesis Pharmaceuticals Enterprises, Inc. "We anticipate that sales of Baobaole chewable tablets will have a significant impact on Company FY2008 revenues."
About Genesis Pharmaceuticals Enterprises
Genesis Pharmaceutical Enterprises, Inc. is engaged in the research, development, production, marketing and sales of pharmaceutical products in the People's Republic of China. Its operations are located in Northeast China in an Economic Development Zone in Laiyang City, Shandong province. Genesis produces tablets, capsules, and granules for both western and Chinese herbal-based medical drugs. The Company maintains a representative office in the U.S. For more information, refer to http://www.Genesis-China.net. Information on the Company's website, or any other website, is not a part of this press release.
Safe Harbor Statement
Certain statements set forth in this press release constitute "forward-looking statements." Such statements are not guarantees of future performance and are subject to risks and uncertainties that could cause the Company's actual results and financial position to differ materially from those included within the forward-looking statements. Forward-looking statements involve risks and uncertainties, including those relating to the Company's ability to introduce, manufacture and distribute new drugs. Actual results may differ materially from anticipated or predicted results, and reported results should not be considered as an indication of future performance. The potential risks and uncertainties include, among others, the Company's ability to obtain raw materials needed in manufacturing, the continuing employment of key employees, the failure risks inherent in testing any new drug, the possibility that regulatory approvals may be delayed or become unavailable, patent or licensing concerns that may include litigation, direct competition from other manufacturers and product obsolescence. More information about the potential factors that could affect the Company's business and financial results is included in the Company's filings, available via the United States Securities and Exchange Commission.
CONTACT: Genesis Pharmaceuticals Enterprises, Inc.
Ms. Elsa Sung, CFO
(877) 895-3650 ext. 701
genesispharm@gmail.com
CCG Elite Investor Relations, Inc.
Mr. Crocker Coulson, President
+1-646-213-1915 (New York)
crocker.coulson@ccgir.com
Optionen
| Antwort einfügen |
| Boardmail an "_bbb_" |
|
Wertpapier:
EUTEX EUROPEAN TELCO
|
0
In reply to: None Date:1/13/2008 9:19:19 AM
Post #of 3025
Dividend
We all know there possibly will be a dividend from the sell of shares SPEH and LTUS If you look at the shares GTEC owns you can find the following numbers (on Yahoo: http://biz.yahoo.com/t/43/671.html )
Lotus 5.399.206
Speedhaull 8.542.500
Value (stock price 10th january 2008)
Lotus 1,00 dollar
Speedhaull 1,25 dollar
that would value these positions at:
Lotus 5.399.206 dollar + Speedhaull 10.678.125
Total 16 million dollar
Divided by approx. 91.000.000 shares (US shareholders of GTEC before merger) would be a maximum dividend of approximately 0.17 dollar !!!! Maybe this explains the action of last friday.
Off course there would be costs that first have to be paid to arrange all this...
Optionen
| Antwort einfügen |
| Boardmail an "_bbb_" |
|
Wertpapier:
EUTEX EUROPEAN TELCO
|
0
Dutton Associates Announces Investment Opinion: Genesis Technology Strong Speculative Buy Rating In Update Coverage By Dutton Associates
16:40 15.01.08
EL DORADO HILLS, Calif.--(BUSINESS WIRE)--
Dutton Associates updates its coverage of Genesis Technology Group (OTCBB:GTEC) with a Strong Speculative Buy rating and a price target of $0.42. The 20-page report by Dutton senior analyst Stanley Ng is available at www.jmdutton.com as well as from First Call, Bloomberg, Zacks, Reuters, Knobias, and other leading financial portals.
We are updating our research coverage of Genesis Pharmaceuticals Enterprises with a rating of Strong Speculative Buy, following its recent corporate restructure. Genesis operations are principally conducted through China-based Laiyang Jiangbo Pharmaceutical Co. Ltd. (Laiyang Jiangbo), is engaged in the research, development, production, marketing and sales of pharmaceutical products in China. Laiyang Jiangbo is one of the major pharmaceutical companies in China producing tablets, capsules and granules for both Western and Chinese herbal-based medical drugs. Laiyang Jiangbos core products include clarithromycin sustained-release tablets, itopride hydrochloride granules, ciprofloxacin hydrochloride tablets, Baobaole chewable tablets and paracetamol tablets. In our view, Genesis offers investors an opportunity to invest in the huge and growing pharmaceutical market in China, the prospects of which are driven by a large population of 1.3 billion, increasing healthcare services due to improving affordability as a result of rapid economic growth and thus increasing affluence, and the Chinese governments policy to broaden the availability of healthcare insurance to help stimulate the demand for better quality healthcare services and drug products. Based on our revenue and net income forecasts, we expect Genesis revenue to increase 26% from $76.2 million in FY2007 to $96.2 million in FY2008 and rise 29% to $123.7 million in FY2009, while net income estimates are $19.3 million in FY2008 and $28.2 million in FY2009.
About Dutton Associates
Dutton Associates is one of the largest independent investment research firms in the U.S. Its 30 senior analysts are primarily CFAs and have expertise in many industries. Dutton Associates provides continuing analyst coverage of over 140 enrolled companies, and its research, estimates, and ratings are carried in all the major databases serving institutions and online investors.
The cost of enrollment in our one-year continuing research program is US $35,000 prepaid for 4 Research Reports, typically published quarterly, and requisite Research Notes. The Firm does not accept any equity compensation. We received $35,000 from the Company for 4 quarterly Research Reports with coverage commencing on 04/27/2007. Our principals and analysts are prohibited from owning or trading in securities of covered companies. The views expressed in this research report accurately reflect the analyst's personal views about the subject securities or issuer. Neither the analyst's compensation nor the compensation received by us is in any way related to the specific ratings or views contained in this research report or note. Please read full disclosures and analyst background at www.jmdutton.com before investing.
Optionen
0
(kleiner Scherz meinerseits)
Die 0,42$ sind (sehr) vorsichtig angesetzt - sollten die Quartalszahlen von GTEC (Pharmaceuticals - nicht mehr GTEC Technicals) [angekündigt für Feb.08] die Erwartungen übertreffen, geht hier "die Post ab"
alles nur meine Meinung...
ax
Optionen
0
Optionen
| Antwort einfügen |
| Boardmail an "_bbb_" |
|
Wertpapier:
EUTEX EUROPEAN TELCO
|
Angehängte Grafik:
gtec_future_prd.jpg
gtec_future_prd.jpg
0
Letzter Vortag Umsatz Veränderung
0,225 € 0,181 € 21.918 € +24,30%
Börsenplatz: Frankfurt Stand: 13:01
Optionen
| Antwort einfügen |
| Boardmail an "_bbb_" |
|
Wertpapier:
EUTEX EUROPEAN TELCO
|
0
Optionen
| Antwort einfügen |
| Boardmail an "_bbb_" |
|
Wertpapier:
EUTEX EUROPEAN TELCO
|
Angehängte Grafik:
waldbrillenfuzzi.jpg

waldbrillenfuzzi.jpg
0
LAIYANG, China, July 23 /Xinhua-PRNewswire-FirstCall/ -- Genesis Pharmaceuticals Enterprises, Inc. (OTC:GTEC) (BULLETIN BOARD: GTEC) ("Genesis" or the "Company"), a leading pharmaceutical company in the People's Republic of China, held a Board of Directors meeting on July 18, 2008 at which a new Board member was appointed to serve on the Board of Directors of the Company, and an Audit Committee and a Compensation Committee were formed.
Mr. Michael Marks was appointed to the Company's Board of Directors on July 18, 2008. Since 2007, he has served as an independent director of China Housing & Land Development, Inc., a property developer in China. In 2006, Mr. Marks became President of Middle Kingdom Alliance Corp., a publicly traded Special Purpose Acquisition Corporation active in China. In 2003, Mr. Marks founded the China practice of Sonnenblick Goldman, a real estate investment bank, and served as its Managing Director in China until 2007. In 2001, he founded B2Globe, providing technology solutions to international internet businesses in Asia. In 1999, he co-founded Metro Corporate Training in Shanghai to offer training and management development, and was its Chief Executive Officer until 2001. During his nine-year tenure in China, Mr. Marks has had a role as advisor, banker or principal in over $2 billion of transactions. From 1998 to 1999, Mr. Marks worked as a management consultant with Horwath Asia Pacific in Australia and China. From 1995 to 1998, Mr. Marks worked in the audit, corporate finance and advisory divisions of PricewaterhouseCoopers in South Africa. Mr. Marks received a Bachelor of Commerce (Honors) in 1994 and Masters of Commerce in 1997 from the University of the Witwatersrand in Johannesburg, South Africa. In 1998, he graduated with a Bachelor of Arts (Psychology) degree from the University of South Africa. In 1997, Mr. Marks became a Chartered Accountant in South Africa, and a Fellow of the Association of International Accountants in the United Kingdom in 1999. He speaks fluent Mandarin, French and English.
The Board of Directors accepted the resignations of Mr. Zhang Yihua and Mr. Rodrigo Arboleda. The resignations of Mr. Zhang and Mr. Arboleda from the Company's Board of Directors were not the result of any disagreements with the Company on any matter relating to the Company's operations, policies or practices.
"I look forward to working with Genesis," stated Michael Marks. "Having participated in the growth of a number of companies in China, I know that Genesis is a company with a tremendous future. I am excited to be a part of its development."
The Board of Directors authorized and approved creation of an Audit Committee that will oversee matters relating to the Company's financial reporting. Qualifying as an "audit committee financial expert" in compliance with applicable SEC and current stock exchange rules and regulations, Michael Marks was appointed as the Chairman of the Audit Committee. Each member of the Audit Committee is "independent" as defined in Section 10A of the Securities Exchange Act of 1934.
The Board of Directors also authorized and approved creation of a Compensation Committee that will, among other things, make recommendations to the Board of Directors concerning salaries and incentive compensation for the Company's officers. Feng Xiaowei was appointed as the Chairman of the Compensation Committee. Each member of the Compensation Committee is "independent" as defined in Section 10A of the Securities Exchange Act of 1934.
"We are pleased to welcome Michael Marks to serve as a member of our Board of Directors. We believe that he will make an immediate and valuable contribution to the work of our Board," said Mr. Cao Wubo, Chairman and CEO of Genesis Pharmaceuticals Enterprises. "At its last meeting, our Board of Directors created an Audit Committee and Compensation Committee composed of independent Board members. We believe that the establishment of these committees will help to assure investors that Genesis is fully committed to the highest possible standards of corporate governance."
About Genesis Pharmaceuticals Enterprises, Inc.
Genesis Pharmaceuticals Enterprises, Inc. is a U.S. public company engaged in the research, development, production, marketing and sales of pharmaceutical products in the People's Republic of China. Its operations are located in Northeast China in an Economic Development Zone in Laiyang City, Shandong province. Genesis is a major pharmaceutical company in China producing tablets, capsules, and granules for both western and Chinese herbal- based medical drugs.
Safe Harbor Statement
Certain statements in this press release that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements are not guarantees of future performance and are subject to risks and uncertainties that could cause the Company's actual results and financial position to differ materially from those included within the forward-looking statements. Forward-looking statements involve risks and uncertainties, including those relating to the Company's ability to introduce, manufacture and distribute new drugs. Actual results may differ materially from anticipated or predicted results, and reported results should not be considered as an indication of future performance. The potential risks and uncertainties include, among others, the Company's ability to obtain raw materials needed in manufacturing, the continuing employment of key employees, the failure risks inherent in testing any new drug, the possibility that regulatory approvals may be delayed or become unavailable, patent or licensing concerns that may include litigation, direct competition from other manufacturers and product obsolescence. More information about the potential factors that could affect the Company's business and financial results is included in the Company's filings, available via the United States Securities and Exchange Commission.
For more information, please contact:
Genesis Pharmaceuticals Enterprises, Inc.
Ms. Elsa Sung, CFO Tel: +1-954-727-8436 Email: Web: http://www.genesis-china.net/
CCG Investor Relations, Inc.
Mr. Crocker Coulson, President Tel: +1-646-213-1915 Email: Web: http://www.ccgir.com/
DATASOURCE: Genesis Pharmaceuticals Enterprises, Inc.
CONTACT: Ms. Elsa Sung, CFO of Genesis Pharmaceuticals Enterprises, Inc.,
+1-954-727-8436, or ; Mr. Crocker Coulson, President of
CCG Elite Investor Relations, Inc., +1-646-213-1915, or
Web site: http://www.genesis-china.net/
http://www.ccgelite.com/
Optionen
| Antwort einfügen |
| Boardmail an "_bbb_" |
|
Wertpapier:
EUTEX EUROPEAN TELCO
|
0
WKN: A0RNJBISIN: US47737R1014 Symbol: JGBO
http://www.ariva.de/..._Pharmaceuticals_ehem_GNPH_A0RNJB_JGBO_t376817
Optionen
| Antwort einfügen |
| Boardmail an "_bbb_" |
|
Wertpapier:
EUTEX EUROPEAN TELCO
|
0



 Thread abonnieren
Thread abonnieren


