Galena Biopharma Announces Publication of Two Abstracts at the 2015 American Society of Clinical Oncology Annual Meeting
Galena Biopharma
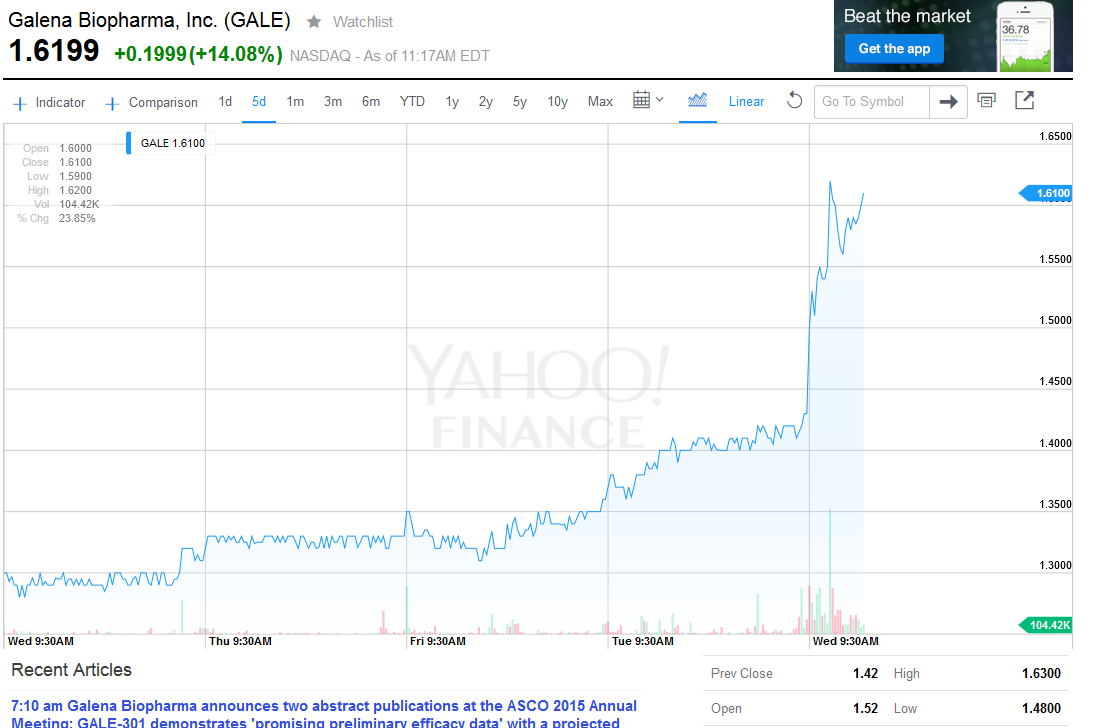
4 hours ago
GlobeNewswire
GALE-301 demonstrates promising preliminary efficacy data with a projected 78% reduction in relative risk of recurrence in the 1000 mcg dose cohort
Leica Biosystem's Bond Oracle HER2 Immunohistochemistry (IHC) System distinguishes HER2 1+ and 2+ expressions and supports its use as a companion diagnostic for NeuVax(TM) (nelipepimut-S)
PORTLAND, Ore., May 27, 2015 (GLOBE NEWSWIRE) -- Galena Biopharma, Inc. (GALE), a biopharmaceutical company developing and commercializing innovative, targeted oncology therapeutics that address major medical needs across the full spectrum of cancer care, today announced two abstract publications at the American Society of Clinical Oncology (ASCO) 2015 Annual Meeting.
"The two abstracts published by ASCO provide meaningful advancements for both of our cancer immunotherapy programs, NeuVax and GALE-301," said Mark W. Schwartz, Ph.D., President and Chief Executive Officer. "The use of Leica's Bond Oracle HER2 IHC system ensures we have enrolled the specified HER2 1+/2+ patients for our Phase 3 NeuVax(TM) PRESENT trial and moves us further down the path of providing targeted, personalized medicine for these women. Meanwhile, the published preliminary data from our GALE-301 program is quite promising as it shows significant reduction of recurrences with our vaccine. We expect to present more mature data at a scientific conference this Fall, and we are currently evaluating the next steps to potentially advance GALE-301 into a randomized late-stage trial to prevent recurrence in ovarian and endometrial cancers."
GALE-301
GALE-301 (E39) is a cancer immunotherapy targeting folate binding protein receptor-alpha to prevent ovarian and endometrial cancer recurrence in the adjuvant setting. In abstract #e14031, entitled, "Preliminary Results of the Phase I/IIa Dose Finding Trial of a Folate Binding Protein Vaccine (E39+GM-CSF) in Ovarian and Endometrial Cancer Patients to Prevent Recurrence," data show that GALE-301 is well tolerated and elicits a strong and dose-dependent in vivo immune response. The trial is designed as a safety and dose optimization trial and is not powered for a disease free survival efficacy endpoint. However, early efficacy results from the trial are promising in the 1000 mcg dose cohort. Of the 51 patients enrolled in the trial, 29 were in the vaccinated group (15 patients at 1000 mcg vs. 14 patients at <1000 mcg) and 22 were in the control group. With 9.8 months median follow-up, the 1000 mcg dose group had only one clinical recurrence vs 11 in the vaccine group (6.7% vs. 50% CG, p = 0.01). Combining all dose groups, the complete response (CR) rate was 38% in the vaccine group vs. 50% in the control group (p = 0.41). Currently, the estimate for disease free survival at two years is 85.7% (1000 mcg dose group) vs. 19.2% for the control group (p = 0.09), for a 78% reduction in relative risk of recurrence. The full abstract can be found here.
NeuVax(TM) (nelipepimut-S) Companion Diagnostic
As part of the Phase 3 PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) trial, Galena implemented central laboratory testing for all potential patients to confirm validated and robust entry criteria, and to ensure the enrollment of the targeted patient population. To improve accuracy and specificity for the HER2 1+ and 2+ status testing, and develop a companion diagnostic for NeuVax, the Leica Bond Oracle(TM) HER2 Immunohistochemistry (IHC) system has been incorporated as central HER2 screening for the PRESENT study.
In abstract #e11609, entitled, "Analytical Validation of BOND Oracle HER2 IHC System for Identifying Low to Intermediate HER2 Expressing Breast Cancer in NeuVax PRESENT Phase 3 Clinical Trial," data demonstrate a direct correlation between cell line receptor load, quantitative measure of HER2 protein, and IHC score. The ability to discriminate HER2 protein expression at the low and intermediate levels in breast cancer tumors will identify patients for new treatments in development such as NeuVax. Specifically, the validation of the Bond Oracle HER2 IHC System to distinguish lower levels of HER2+ expressions supports its use as a companion diagnostic. The full abstract can be found here.
About GALE-301 (Folate Binding Protein)
GALE-301 (Folate Binding Protein (FBP)) is a cancer immunotherapy targeting the prevention of cancer recurrence in the adjuvant setting. GALE-301 targets folate binding protein receptor-alpha, a well-validated therapeutic target, is highly over-expressed in ovarian, endometrial and breast cancers. FBP is the source of immunogenic peptides that can stimulate cytotoxic T lymphocytes (CTLs) to recognize and destroy FBP-expressing cancer cells. GALE-301 consists of the FBP peptide(s) combined with the immune adjuvant, granulocyte macrophage-colony stimulating factor (GM-CSF). Galena has completed enrollment in a Phase 2a trial with GALE-301 in two gynecological cancers: ovarian cancer and endometrial adenocarcinomas (clinicaltrials.gov identifier: NCT01580696).
Related Quotes
|
Angehängte Grafik:
gale_news.png (verkleinert auf 45%)

 1 |
|
3 |
4 |
5 |
6 |
7 |
8 |
9 |
...
| 11
1 |
|
3 |
4 |
5 |
6 |
7 |
8 |
9 |
...
| 11


 Thread abonnieren
Thread abonnieren


